EU PFAS Restriction: PPWR Packaging Updates + RoHS Exemptions Deadline
EU PFAS restriction planning is accelerating ahead of the 2026 consultation: ECHA is collecting evidence on substances in packaging under PPWR, preparing the PFAS restriction process (info session 30 Oct 2025), and finalizing RoHS lead exemption renewals with new deadlines (31 Dec 2025 / 30 Jun 2026) and expiry dates through 2027.
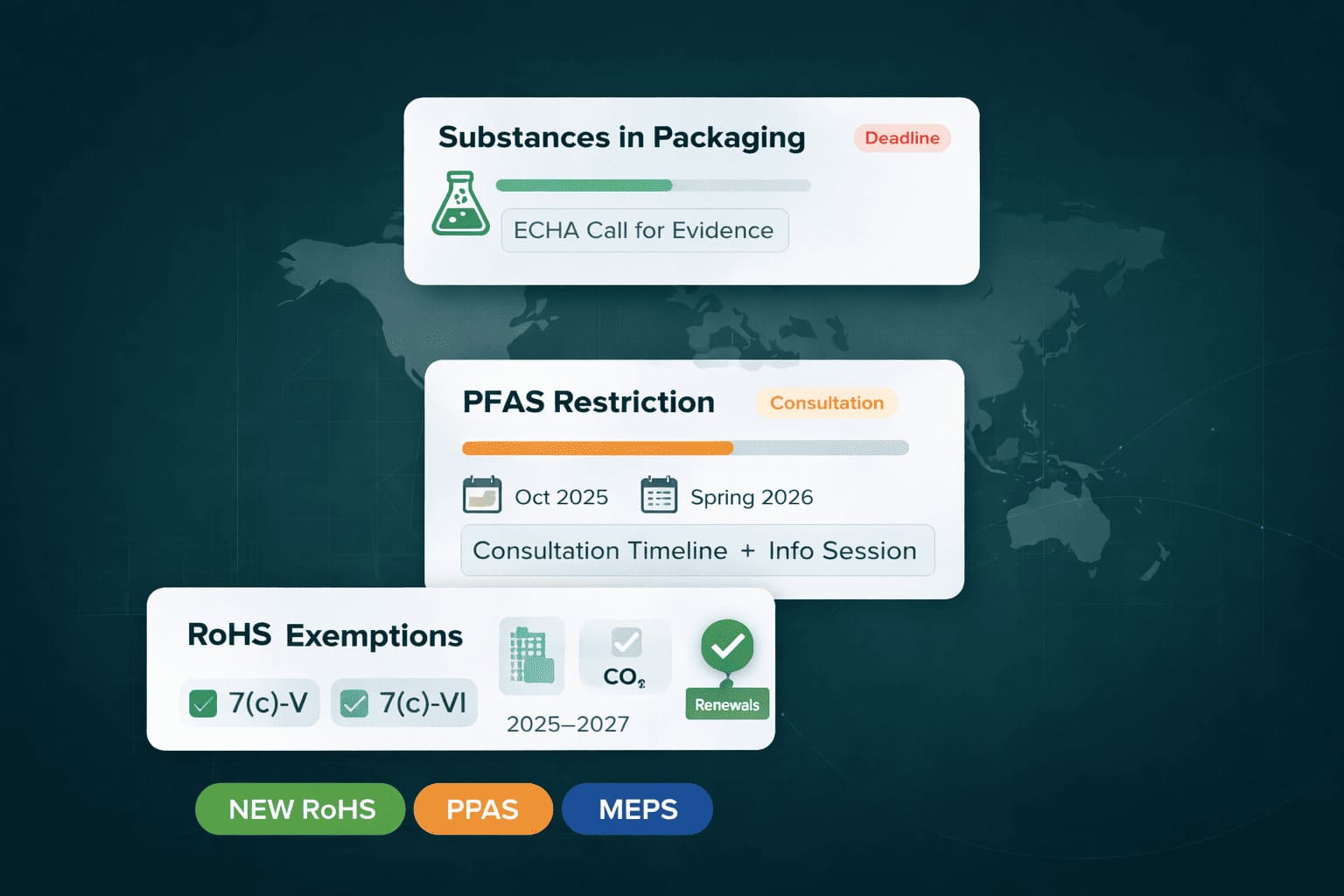
Table of Contents
Three significant regulatory developments in the EU now directly impact packaging materials, PFAS restrictions, and RoHS exemptions. Here's a breakdown of what’s changing and how manufacturers should prepare.
🇪🇺 PPWR: ECHA Call for Evidence on Substances in Packaging (Deadline Oct 2025)
Original Regulation:
ECHA has launched a Call for Evidence to gather information for a working paper on the Packaging and Packaging Waste Regulation (PPWR), which came into effect on February 11, 2025, replacing the previous directive.
The focus is on packaging, materials used, packaging waste, waste management, and recycling technologies. The regulation aims to reduce packaging use and waste, minimize primary raw material consumption, and support the transition to a circular, sustainable economy.
Statut: In effect
This call for evidence is addressed to interested parties, such as companies (or trade associations) across the entire packaging supply chain, including manufacturers, importers, distributors, formulators, end-users of packaging and packaging components, waste management practitioners, and recyclers. Other stakeholders (e.g., scientific organizations, NGOs, Member State Authorities, and international organizations) are invited to participate by uploading written submissions or documents containing relevant information.
Deadline for providing input: October 28, 2025.
📌 What This Means for Manufacturers
- Audit your product packaging materials and any components potentially affected by substance-level scrutiny.
- Prepare scientific, technical, or commercial input now for submission before the October 28 deadline.
- Coordinate responses across regulatory, procurement, and sustainability teams — input from manufacturers will shape future substance restrictions.
🇪🇺 EU PFAS Restriction: Consultation Timeline + Info Session (Oct 2025 / Spring 2026)
Original Regulation:
ECHA plans to launch a consultation on the draft opinion of the Committee for Socio-Economic Analysis (SEAC) on the proposal for an EU-wide restriction of perfluoroalkyl and polyfluoroalkyl substances (PFAS), following the Committee meeting in March 2026. ECHA will hold an online information session on 30 October 2025 to support interested parties in preparing for the consultation.
The proposal to restrict PFAS in the EU/EEA was prepared by the authorities of Denmark, Germany, the Netherlands, Norway, and Sweden and submitted to ECHA on 13 January 2023. The proposal aims to reduce PFAS emissions into the environment and make products and processes safer for humans.
Statut: In effect The consultation is open to all interested parties, including industry representatives, civil society organisations, researchers, and the public.
📌 What This Means for Manufacturers
- Identify and map all PFAS use across product lines — prepare justifications or alternatives.
- Attend the 30 October 2025 online info session to understand evidence expectations.
- Start cross-functional impact assessments to inform your formal submission once the consultation opens.
🇪🇺 RoHS Exemptions: Renewals, Split IDs (7(c)-V/VI), and New Deadlines (2025–2027)
Original Regulation:
The European Commission has adopted the final RoHS Delegated Directives regarding lead exemptions. Translations into all EU languages have already been completed, so the directives are expected to be published in the Official Journal of the EU shortly.
As anticipated, the draft expiry date originally set for 31 December 2026 has been extended to 30 June 2027. However, the expiry date of 31 December 2027 for the exemptions under 7(c)-II and the split exemptions remains unchanged. Additionally, the exemption previously classified as 7(c)-I has now been divided into 7(c)-V and 7(c)-VI, while 7(a) has been split into 7(a)-I through 7(a)-VII.
Statut: Active enforcement The deadlines for renewal applications are as follows:
- 30 June 2027 has now been moved to 31 December 2025.
- 31 December 2027 has now been moved to 30 June 2026.
📌 What This Means for Manufacturers
- Immediate action needed: update compliance documentation for exemptions impacted by split and reclassification.
- Coordinate with supply chain partners to ensure awareness of new expiry dates and exemption IDs.
- Submit renewal applications before the revised deadlines: 31 December 2025 and 30 June 2026.
✅ Need to integrate these shifts into your product compliance workflow? At EcoComply, we monitor EU regulatory changes in real time and translate them into clear, actionable tasks for your operations, legal, and R&D teams.
Frequently Asked Questions
Everything you need to know about EU compliance

Launch in the EU without compliance guesswork
Get a clear view of what documents you need, what’s missing, and how to avoid market access blockers, built for electronics & IoT manufacturers.
- Identify missing CE deliverables (DoC, test reports, technical file)
- Plausibility checks aligned with market surveillance expectations
- Expert validation for edge cases